Abstract
Introduction: Severe graft-versus-host disease (GvHD) is a major barrier to the success of haploidentical stem cell transplantation (haplo-SCT). However, there are no standard second-line treatment for steroid-resistant (SR) acute graft-versus-host disease (aGVHD) and causes poor overall survival (OS) in haplo-SCT recipients. Mesenchymal stromal cells (MSCs) may be a potential efficacious therapy for SR aGVHD.
Methods: We conducted a multicenter, randomized, open-label trial to evaluate the efficacy and safety of MSCs combined with basiliximab as second-line treatment for haplo-SCT recipients with SR aGVHD. In both treatment groups, basiliximab was administered on days 1, 3, and 8, and repeated weekly until aGVHD was less than grade II, or showed no response after four doses. For patients assigned to the MSC group, MSCs were given in addition to basiliximab at a dose of 1.0×106/kg weekly for 4 weeks as a cycle, and further infusions of MSCs were based on the response of MSC. The primary endpoint was the rate of aGVHD complete response (CR) in week 4 after randomization. The key secondary endpoints were OS at the end of weeks 4/8/12/24/52 and the partial response (PR) rate in week 4. Safety analyses included evaluations of infusion toxicity, infections, hematologic toxicity, etc., and tumor relapse.
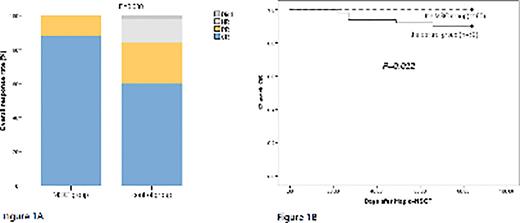
Results: From February 2021 to May 2022, a total of 100 patients with SR aGVHD after haplo-SCT were enrolled and randomized 1:1 to receive MSCs combined with basiliximab (MSC group, n=50) or basiliximab alone (control group, n=50) in 3 centers and all the enrolled patients were included in intention-to-treat (ITT) analysis. Among patients in MSC group, 3 withdrew informed consent and 3 discontinued MSC treatment before completing 4 MSC infusions due to patients’ decision after randomization, and so were not included in the safety analysis set. In the ITT population, the median age was 32 years (range, 18-68), consisting 49 male patients. The median time from aGVHD diagnosis to basiliximab therapy was comparable between the two groups (4 days vs. 5days, P=0.92). The proportion of patients with grade 2-4 SR aGVHD or grade 3-4 SR aGVHD was comparable between the MSC group and the control group (82.0% (41/50) vs. 86.0% (43/50), P=0.585;38.0% (19/50) vs. 44.0% (22/50), P=0.542). For the primary endpoint, the rate of aGVHD CR in week 4 after randomization was significantly higher in the MSC group than in the control group (84.0% (42/50) vs. 60% (30/50), P=0.008) (Figure 1). Patients in the MSC group received higher 4-week overall response rate (ORR) comparing to patients in the control group (96.0% (48/50) vs. 83.0% (41/50), P=0.025) (Figure 1A). However, for CR or ORR at week 8, week 16 and week 52, there was no significant difference between the two groups. In the ITT population, patients received the median of four doses of basiliximab (range, 2 to 8).And less patients in the MSC group used ≥ 6 doses of basiliximab (2%(1/50) vs. 16% (8/50), P=0.008).The 12-week OS was significantly higher in the MSC group than in the control group (P=0.022). MSCs were well tolerated, and no infusion-related toxicity was observed. There was no difference between the MSC and control groups in terms of infections, hematologic toxicity, or tumor relapse. Four patients in the MSC group and 5 patients in the control group died because of GVHD progression (n = 1), severe infection (bacterial central nervous system infection: n = 1; fungal pneumonia: n = 2), and poor graft function (n = 1).
Conclusions: MSCs plus basiliximab is a safe and effective approach for treating SR aGVHD in patients who receive haplo-SCT, decreases the dose of basiliximab without increasing relapse, is well-tolerated and improves patients’ prognosis.
Figure Legends: Figure 1A. A Overall response (OR) in week 4 after randomization; Figure 1B. The 12-week OS was significantly higher in the MSC group
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal